Mechanical Properties of Elastomers

One of the distinguishing properties of elastomers is their high elasticity. The main characteristics of elastomers present themselves, in particular when comparing them to steel. Below we would like to elaborate on the main properties of elastomers in more detail. Figure 1 shows the main characteristics of elastomers. They are able to combine spring properties as well as damping ability in one component.
Elasticity of elastomers
According to the most significant property of elastomers is their high elasticity. This primarily refers to their ability to recover their original state with no sensible time delay after being stretched, compressed or deformed in any other way, when the deforming force is removed.
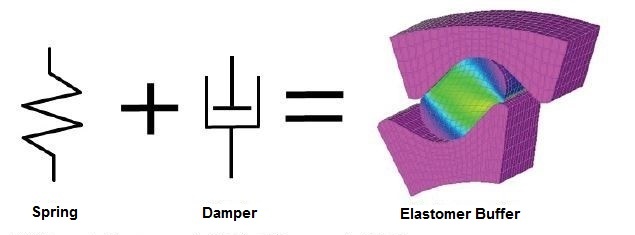
Figure 1
Apart from that, elastomers feature a very high resilience which is about 400 times larger than that of steel. Compared to rubber, steel consists of metallic atoms arranged in a lattice, whereas elastomers consist of long threadlike molecules. When in the state of rest, these molecular chains are not oriented in a straight line, but take a coil-type state. When an external force is applied, these random coils untangle and the chains orient and align into a more ordered state.
The end-to-end distance of the molecules may then increase by several hundred per cent. This accounts for the high resilience of elastomers. When the force is removed, the molecules immediately return to the coil-type state. This phenomenon can be explained with the second law of thermodynamics:
ds = (dq + dwr) T
According to this law, an ordered system by itself returns to a disordered system. In this case, the stretched molecule chain does not maintain its high degree of order in an almost straight arrangement, but seeks for the disordered state of random coils.
Because entropy is figuratively the measure for disorder, rubber elasticity is referred to as entropic elasticity. When stressed molecules are also strained, this additional strain is referred to as energetic elasticity. The respective elasticity ranges are depicted in Figure 2. The transition from the entropic elasticity to energetic elasticity is assumed to be the inflection point in the stress/strain diagram.
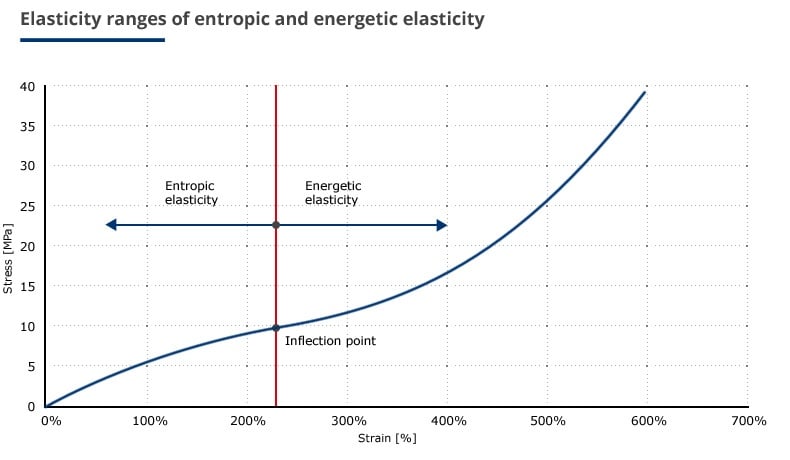
Figure 2
Visco-elastic behavior
As illustrated in Figure 1, an elastomer is a so-called Kelvin Body. This means that the rubber elasticity can be regarded as a model of an ideal spring with a parallel connected damping element. Damping must be considered, in particular for dynamically loaded materials. High internal friction in a material while it undergoes periodic deformation will cause heat, and this may eventually lead to the destruction of a component [GK75].
Deformation not only involves elastic deformation, but plastic deformation as well. Plastic deformation may either be caused by the fact that the molecular coils change their inner arrangement when an external force is applied, or that the sulfur cross-links detach and tie up again in other low-strain areas. Another possible development is that caoutchouc chains may slide off from the surface of the fillers. As a result, an elastomer piece will not fully return into its initial position and some degree of change in shape will remain. On one hand, this depends on the composition of the mixture, and on the other hand on the degree of deformation, duration of deformation and temperature.
Such behavior brings to mind: viscous liquid. Therefore, elastomers are also called visco-elastic. One parameter that measures internal damping is the impact performance.
Thermal behavior of elastomers
The service temperature has an important influence on the elasticity of an elastomeric material. The elasticity increases with rising temperature. The reason for the increasing elasticity is the increase of the available energy to deform the molecules. When the temperature decreases, it may reach such a low level that the energy will no longer be sufficient for the required mobility.
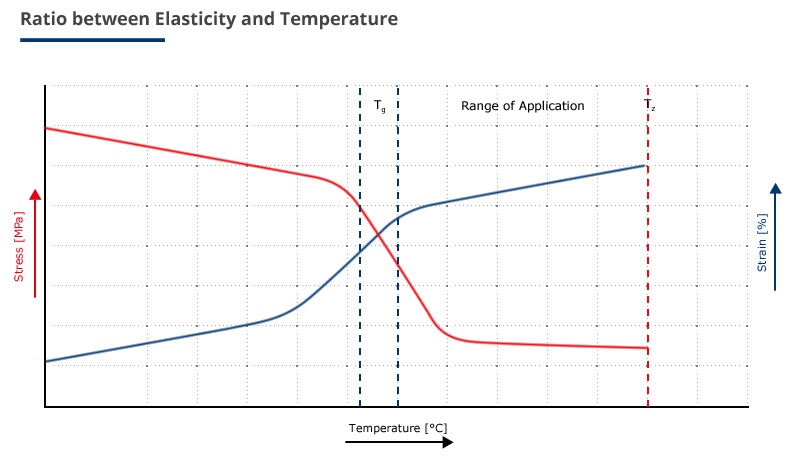
Figure 3
Glossary
Energy elasticity: describes the plastic deformation / plastic strain of materials. Energy elasticity occurs for elastomers when the range of entropy elasticity has been exceeded due to external stresses.
Entropy: Physical term in thermodynamics, characterizing thermal processes.
Entropy elasticity: or rubber elasticity, describes the urge of molecules to return to the original state after a mechanical load.
Viscoelasticity: Describes a material behavior that is both elastic (returns in the original state after loading) and viscous (the elongation remains completely after relief). This behavior depends on the time, temperature and frequency of the load.
Sources
S. Graf; Untersuchungen an seriell angeordneten Elastomerelementen in Wellenkupplungen mittels der Finite Elemente Methode, Master Thesis, Beuth Hochschule für Technik, Berlin 2015
[GK75] W. Gohl and G. Kolb. Elastomere - Dicht- und Konstruktionswerkstoffe. Lexika Verlag, Würzburg, 2nd edition, 1975.


Comment